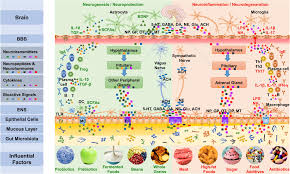
The bacteria in the microbiome help digest our food, regulate our immune system, protect against other bacteria that cause disease, and produce vitamins including B vitamins B12, thiamine and riboflavin, and Vitamin K, which is needed for blood coagulation.
Additionally, our microbiome is essential in the innate and adaptive immunity and in turn, the immune system shapes the microbiome.
We are synergy in motion, each system interdependent, mind, body and spirit creating the whole of our entity.
In as much as our microbiome influences our digestion, assimilation and elimination, it is also bidirectionally influenced. Our body is in a constant state of flux, with each system maintaining its own biochemical feedback to and from organs, cells & tissues to maintain equilibrium.
When the microbiome is our of balance it has a detrimental effect on all our systems. It is a keystone marker for health and well-being and must be kept in pristine condition.
There are so many detrimental influences to upset this delicate balance, from dietary indiscretions, genetic polymorphisms, to bacterial infections and environmental assaults, the list is endless.
We can maintain a healthy microbiome, especially when we educate ourselves in its function.
I rely heavily on herbal medicine, but also on food based supplements that have proven to support the expression of health protecting genes in every cell of the body.
These supplements significantly activate cellular defences providing core upstream cellular defence mechanisms which induce glutathione production, primary antioxidant enzymes, detoxification enzymes, provide anti-inflammatory actions and normalise the microbial populations in the gut and respiratory membranes.
Additionally, I offer nutritional guidance along with traditional naturopathic principles to guide your health journey to wellness. There is a plethora of information at our fingertips that, along with extensive study and research we can implement and work together to bring about cellular changes to restore your health.
Studies show that the gut makes most of the important chemicals that affect memory. Often called “the second brain,” the gut houses the largest colony of microorganisms that regulate digestion, immunity, vitamin production and, yes, memory.
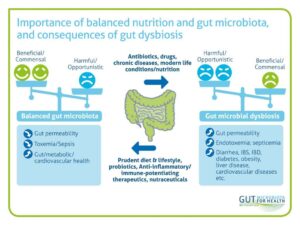
A dense and diverse microbial community inhabits the gut and many epithelial surfaces. Referred to as the microbiota, it has co-evolved with the human host and is beneficial for many host physiological processes.
Gut dysbiosis, or the disruption of the gut microbiota is known to be influenced by host genetics, diet, antibiotics, and inflammation, and it is closely linked to the pathogenesis of inflammatory diseases, such as obesity and inflammatory bowel disease (IBD).
Known as your gut microbiome, microbes are microorganisms, especially bacterium causing disease or fermentation, play a vital role in how you digest food and absorb nutrients, and influence your metabolism, body weight, immune system, and general health. They also influence your brain function.
Obesity is considered a multi-causal and complex disease influenced by factors intrinsic and extrinsic to the individual, such as environmental, genetic, neuronal, endocrine and behavioral components.
Furthermore, overweight and obesity are risk factors for other chronic diseases, such as diabetes mellitus II, cardiovascular diseases and some types of cancer.
The following studies highlight the importance of the microbiota and the various roles played in systemic health.
 The human gut microbiota interactions effect human health during an entire lifetime
The human gut microbiota interactions effect human health during an entire lifetime
It is thought that an infants gut is nearly sterile prior to birth, whereupon the gut is immediately colonised at birth, driven in part by the ingestion of mother’s milk but also due to genetics, microbial exposure from the mother at delivery and antibiotic use
It is proposed that there are d stages of microbial gut colonisation, in an infant from 3 months to 4 years of age. This consists of a developmental phase from 3 – 14 months, followed by a developmental phase from 15 – 30 months, thereafter a stable phase of 31 months and onward. This is, of course dependent upon a host of factors, that we will explore.
A disruption, or shift in the balance of the microbiota results in dysbiosis, caused through the imbalance of the microflora residing in the gut. The most dominant, beneficial consensual gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with the two phyla Firmicutes and Bacteroidetes representing 90% of gut microbiota.
These microbes all play a part in our cellular metabolism.
 The importance of a healthy, balanced microbiome impacts every system of our body
The importance of a healthy, balanced microbiome impacts every system of our body
This study investigates the correlation between the microbiome and Type II diabetes and the interaction of microbial metabolites and alpha lipoic acid, a powerful antioxidant.
It also highlights the interplay of the metabolic transformation crosstalk, whereby the metabolites increase the uptake of the antioxidant, thereby reducing ROS and the consumption of alpha lipoic acid increased the abundance of the bacteria.
It may therefore follow that this crosstalk in the microbiota may well be applicable to nutrient uptake and assimilation
An example here would likely be the production of folate (B9) in the intestine from the consumption of spinach, or the production of butyrate and short chain fatty acids from the consumption of fibre.
We are complex beings that survive the best when we focus on ourselves as synchronistic beings, with multiple systems, working together in harmony.
The study is included for further reading
Enjoy
“Conclusions
There may be a mutual regulatory network between intestinal bacteria and fecal metabolites in T2DM.The increased abundance of Phascolarctobacterium may increase alpha-linolenic acid uptake, and alpha-linolenic acid may also increase the abundance of intestinal Phascolarctobacterium in vivo after metabolic transformation.The combination of the two may play an important role in the treatment of diabetes.”

https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9622494/#!po=0.462963
 The role of the gut axis with various body systems.
The role of the gut axis with various body systems.
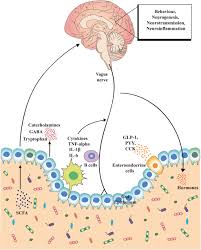
Gut-Brain Axis
Neurologically active compounds produced by the gut microbiome affect brain function by regulating production, metabolism and transmission of neurotransmitters.
Microbes in the gut metabolise the amino acid tryptophan into serotonin and other metabolites, regulating serotonin levels in the brain.
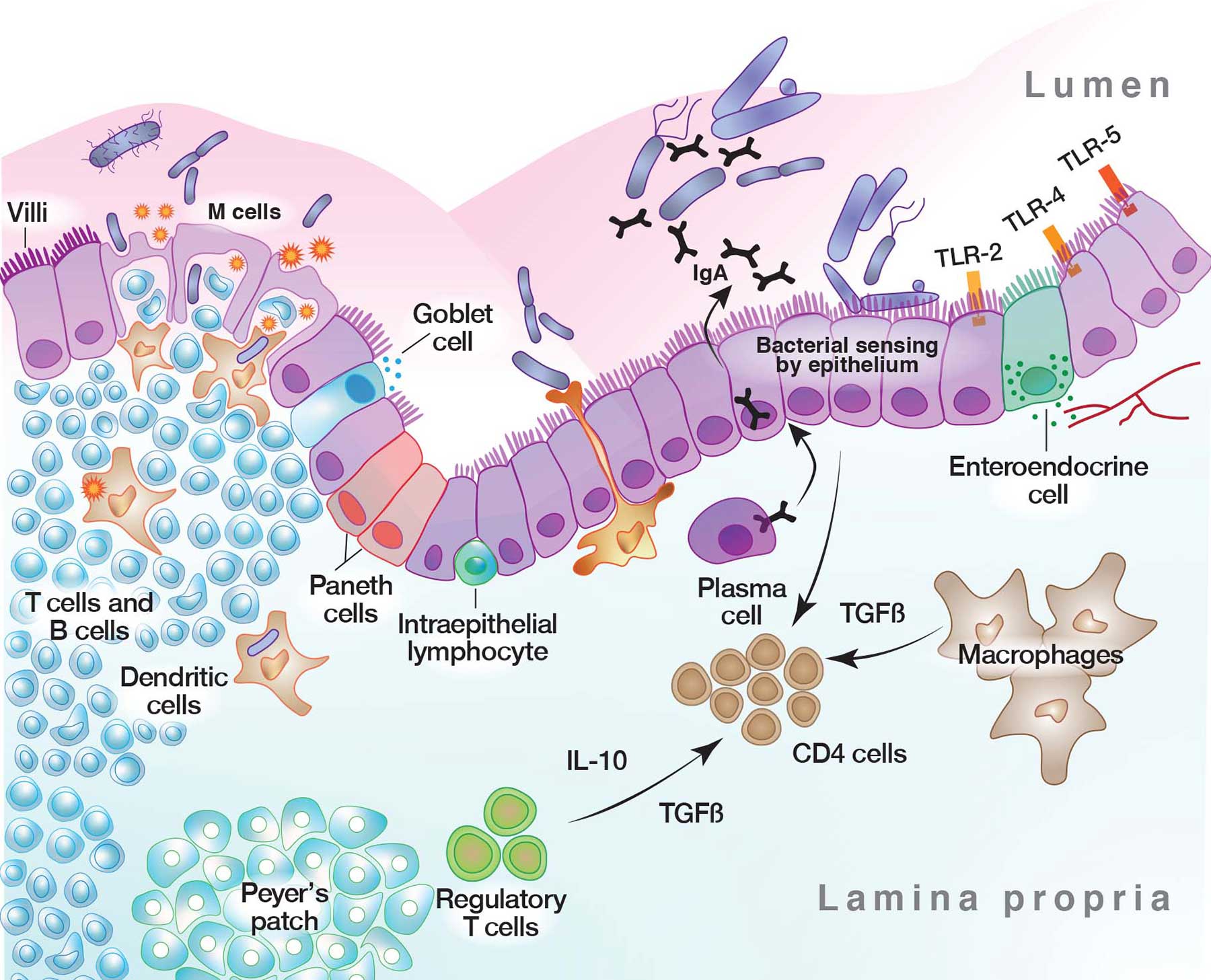 The importance of of the integrity of the gut epithelium along with a healthy microbial population cannot be understated.
The importance of of the integrity of the gut epithelium along with a healthy microbial population cannot be understated.
A dense and diverse microbial community inhabits the gut and many epithelial surfaces. Referred to as the microbiota, it has co-evolved with the human host and is beneficial for many host physiological processes.
A major function of these symbiotic microorganisms is protection against pathogen colonisation and overgrowth of indigenous pathogenic organisms, with dysbiosis or dysfunction of the normal microbial community increasing the the risk of pathogen infection and overgrowth of harmful pathogenic organisms.
The protective mechanisms conferred by the microbiota are complex and include competitive microbial–microbial interactions and induction of host immune responses. Pathogens, in turn, have evolved multiple strategies to subvert colonisation resistance conferred by the microbiota.Understanding the mechanisms by which microbial symbionts limit pathogen colonisation aids the guidance of clinical, therapeutic approaches to prevent or treat disease.
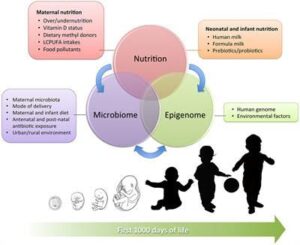 Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-term Health Development
Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-term Health Development
A number of antenatal and postnatal factors, such as maternal and neonatal nutrition, pollutant exposure, and the composition of microbiota, contribute to the establishment of epigenetic changes that can not only modulate the individual adaptation to the environment but also have an influence on lifelong health and disease by modifying inflammatory molecular pathways and the immune response.
Epigenetic modifications are among the most important mechanisms by which environmental factors can influence early cellular differentiation and create new phenotypic traits during pregnancy and within the neonatal period without altering the deoxyribonucleic acid sequence. A number of antenatal and postnatal factors, such as maternal and neonatal nutrition, pollutant exposure, and the composition of microbiota, contribute to the establishment of epigenetic changes that can not only modulate the individual adaptation to the environment but also have an influence on lifelong health and disease by modifying inflammatory molecular pathways and the immune response.
Postnatal intestinal colonisation, in turn determined by maternal flora, mode of delivery, early skin-to-skin contact and neonatal diet, leads to specific epigenetic signatures that can affect the barrier properties of gut mucosa and their protective role against later insults, thus potentially predisposing to the development of late-onset inflammatory diseases.
 Microbiome and Memory
Microbiome and Memory
Our microbiome may play a key role in many of the best-described pathways involved in memory. It has been recently demonstrated that memory, and more specifically forgetting, may be controlled by the actions of microglia
Studies show that the gut makes most of the important chemicals that affect memory. Often called “the second brain,” the gut houses the largest colony of microorganisms that regulate digestion, immunity, vitamin production and, yes, memory.
The study of the gut microbiota-brain axis has become an intriguing field, attracting attention from both gastroenterologists and neurobiologists. The hippocampus is the center of learning and memory, and plays a pivotal role in neurodegenerative diseases, such as Alzheimer’s disease (AD). Previous studies using diet administration, antibiotics, probiotics, prebiotics, germ-free mice, and fecal analysis of normal and specific pathogen-free animals have shown that the structure and function of the hippocampus are affected by the gut microbiota.
One hundred years ago, the Nobel Prize winner Elie Metchnikoff proposed that cognitive decline and senility might be delayed by manipulating the intestinal microbiome with host-friendly bacteria (Scott et al., 2017).
in both Alzheimer’s Disease patients and AD model animals, significant changes in the gut microbiota have been reported, some of which increased while others decreased.
Altered gut microbiota in AD patients and model mice. Some differences have been noticed regarding the changes of gut microbiota in AD patients or mouse models. For example, Bacteroidetes and Firmicutes decreased (left) at Phylum level while Actinobacteria, Betaproteobacteria, and Proteobacteria increased (right).
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7873527/
 Focus on the health of the microbiome and obesity
Focus on the health of the microbiome and obesity
Following a sedentary, western lifestyle disrupts the balance of the diversity of the microbiome with deleterious effects not only on our digestion but on every system in our bodies, including metabolic balance.
“Intestinal microbiota has been shown to be a potential determining factor in the development of obesity
The Western Diet, characterized by a large consumption of processed products, saturated fats, sugars and a low fibre content, together with an increasingly sedentary lifestyle, has generated tripled levels of obesity in the world as compared to the year 1975, according to the World Health Organization.
Obesity is considered a multi-causal and complex disease influenced by factors intrinsic and extrinsic to the individual, such as environmental, genetic, neuronal, endocrine and behavioral components.
Furthermore, overweight and obesity are risk factors for other chronic diseases, such as diabetes mellitus II, cardiovascular diseases and some types of cancer.
People with overweight or obesity have been shown to have a specific IM profile, characterized by dysbiosis (imbalance) and lower microbial diversity compared to people with normal weight [6,9]. In this sense, a decrease has been seen in some bacterial phyla, such as the relationship between Bacteroidetes/Firmicutes [10,11], with lower proportions of Bacteroidetes and higher proportions of Firmicutes than those from people without obesity. “
An excessive hyper-inflammatory response-caused septic shock is a major medical problem that is associated with pathogenic bacterial infections leading to high mortality rates.
The intestinal #microbiota and the associated elaborated metabolites such as #scfa – short chain fatty acid #Butyrate have been shown to relieve pathogenic bacterial-caused acute inflammation.
#Butyrate can down-regulate inflammation by inhibiting the growth of pathobionts, increasing mucosal barrier integrity, encouraging obligate anaerobic bacterial dominance and decreasing oxygen availability in the gut.
Butyrate can also decrease excessive inflammation through modulation of immune cells such as increasing functionalities of M2 macrophages and regulatory T cells and inhibiting infiltration by neutrophils.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8540110/#B12-nutrients-13-03627
 Inflammation and the microbiota
Inflammation and the microbiota
Rheumatoid arthritis and spondyloarthropathy are the most common inflammatory rheumatic diseases. As the human microbiome is involved in the immune homeostasis, or balance, it has the potential to be a major factor in the development of autoimmune diseases and rheumatic diseases.
Animal and human translational research suggests an important function of the microbiome in the modulation of the host’s immunity. Although disruptions in the local microbiome composition, commonly referred to as dysbiosis, has been noted in a systemic inflammatory response, generally the development of an inflammatory disease is generally seen as a disturbance of the complex network of interactions between the microbiome, genetic factors and the environment.
A disrupted gut environment is proposed to be associated with dysregulation of the immune response and/or altered dendritic cell function in spondyloarthropathies. (SpA)
Various overlapping mechanisms indicate that microbiota play a significant role in SpA, including alteration of intestinal permeability (possibly determined by genetic factors such as HLA-B27 expression), decrease in production of anti-inflammatory metabolic products, activation of intestinal immunity, and migration of microbial products to peripheral joints, leading to local immune activation.
As more human microbiome studies become available, a greater understanding of mucosal immunity and the host immune responses is being achieved. Various dysbioses in the microbiota of patients are being identified. Pathogenic, microbiota related activation of intestinal Th17 and Th9 cells are commonly implicated in the hypotheses of immune dysregulation commonly associated with SpA along with molecular mimicry with mucosal bacteria. It has been proposed in the light of these findings that clinical intervention by way of dietary modifications, functional foods, probiotics may hold promise.
https://www.frontiersin.org/articles/10.3389/fcimb.2020.491160/full
 The gut microbiota, an integral part of the human body, comprise bacteria, fungi, archaea, and protozoa.
The gut microbiota, an integral part of the human body, comprise bacteria, fungi, archaea, and protozoa.
Gut dysbiosis, or the disruption of the gut microbiota is known to be influenced by host genetics, diet, antibiotics, and inflammation, and it is closely linked to the pathogenesis of inflammatory diseases, such as obesity and inflammatory bowel disease (IBD).
Macrophages are the key players in the maintenance of tissue homeostasis by eliminating invading pathogens and exhibit extreme plasticity of their phenotypes, such as M1 or M2, which have been demonstrated to exert pro- and anti-inflammatory functions.
Macrobiotic-derived metabolites, short-chain fatty acids (SCFAs) and Gram-negative bacterial lipopolysaccharides (LPS), exert anti-inflammatory or pro-inflammatory effects by acting on macrophages. Understanding the role of macrophages in gut macrobiotic-inflammation interactions might provide us a novel method for preventing and treating inflammatory diseases.
Influence of gut microbiota on eye diseases:
 Recently, there has been increasing interest in the interaction between eye and gut microbiota research in ophthalmology.
Recently, there has been increasing interest in the interaction between eye and gut microbiota research in ophthalmology.  The role of the microbiome is slowly beginning to emerge.
The role of the microbiome is slowly beginning to emerge. 
The studies we reported here attest that ocular and extraocular microbiota contribute to some ophthalmic diseases.
These studies confirmed the presence of a gut–eye axis, ocular infections, and inflammatory conditions. The mechanisms underlying these associations have recently become clearer
Emerging findings also suggest the existence of a gut-eye axis, wherein gut dysbiosis may be a crucial factor influencing the onset and progression of multiple ocular diseases, including uveitis, dry eye, macular degeneration, and glaucoma.
The intestinal microbiota is now considered as a complex and dynamic ecosystem that contributes to the proper functioning of body’s immune system and maintenance of the health state.
The composition of microbiota has been found to be mainly dominated by Proteobacteria, followed by Actinobacteria, Firmicutes, and Bacteroidetes.
Several studies have found that various eye diseases are associated with gut and dysbiosis, broadly defined as an intestinal microbial imbalance in the composition of resident commensal communities relative to the community found in healthy individuals.
Hypomethylation of DNA factors discovered in the retinas and retinal pigment epithelium (RPE)–choroidal tissues of EAU mice was associated with an increased production of Th1/Th17-specific cytokines [interferon (IFN)-γ and interleukin (IL)-17] .
Two variants of bacteria have been reported to be associated with POAG and alterations of the intestinal bacterial flora, a possible relationship between glaucoma and Helicobacter pylori [36,38]. More precisely, a higher rate of H. pylori infection was found in patients with glaucoma and the normal tension controls particularly with respect to the relative abundances of Bacteroides and Prevotella.
Additionally, a possible relationship between glaucoma and Helicobacter pylori. More precisely, a higher rate of H. pylori infection was found in patients with glaucoma and the normal tension controls. High-glycaemic index foods are also thought to contribute to higher incidences of retinal changes.
Other researchers have suggested that the bacteria in the mouth and gut can affect the development of glaucoma.
https://doi.org/10.1080%2F07853890.2021.1925150
 Understanding the role of the microbiota and inflammation
Understanding the role of the microbiota and inflammation
Understanding the role of the microbiota and inflammation offers we clinicians a perfect opportunity to assist people who suffer auto-immunity, fatty liver disease, endometriosis, Type 2 diabetes mellitus, Type 1 diabetes mellitus, Inflammatory bowel diseases (IBD), Asthma, Rheumatoid arthritis and other spondyloarthropathies. obesity and so much more.
#autoimmune #microbiota #microbiome
#inflammation #SCFA #Butyrate #Macrophages
“The disruption of the gut microbiota (termed “gut dysbiosis”) is influenced by host genetics, diet, antibiotics, and inflammation, and it is closely linked to the pathogenesis of inflammatory diseases, such as obesity and inflammatory bowel disease (IBD). Macrophages are the key players in the maintenance of tissue homeostasis by eliminating invading pathogens and exhibit extreme plasticity of their phenotypes, such as M1 or M2, which have been demonstrated to exert pro- and anti-inflammatory functions. Macrobiotic-derived metabolites, short-chain fatty acids (SCFAs) and Gram-negative bacterial lipopolysaccharides (LPS), exert anti-inflammatory or pro-inflammatory effects by acting on macrophages. Understanding the role of macrophages in gut macrobiotic-inflammation interactions might provide us a novel method for preventing and treating inflammatory diseases”
 Effects of Symbiotics and Probiotics on Weight Management
Effects of Symbiotics and Probiotics on Weight Management
Following a sedentary, western lifestyle disrupts the balance of the diversity of the microbiome with deleterious effects not only on our digestion but on every system in our bodies, including metabolic balance.
“Intestinal microbiota has been shown to be a potential determining factor in the development of obesity
The Western Diet, characterized by a large consumption of processed products, saturated fats, sugars and a low fibre content, together with an increasingly sedentary lifestyle, has generated tripled levels of obesity in the world as compared to the year 1975, according to the World Health Organization.
Obesity is considered a multi-causal and complex disease influenced by factors intrinsic and extrinsic to the individual, such as environmental, genetic, neuronal, endocrine and behavioral components.
Furthermore, overweight and obesity are risk factors for other chronic diseases, such as diabetes mellitus II, cardiovascular diseases and some types of cancer.
People with overweight or obesity have been shown to have a specific IM profile, characterized by dysbiosis (imbalance) and lower microbial diversity compared to people with normal weight [6,9]. In this sense, a decrease has been seen in some bacterial phyla, such as the relationship between Bacteroidetes/Firmicutes [10,11], with lower proportions of Bacteroidetes and higher proportions of Firmicutes than those from people without obesity. “
From the analysed randomized clinical trials, this systematic review indicates that both probiotics and synbiotics, specifically certain strains of Lactobacillus gasseri, L. rhamnosus, L. plantarum, L. curvatus associated with other Lactobacillus species and/or with species from the Bifidobacterium genus, have the potential to aid in weight and fat mass loss in overweight and obese populations.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8540110/#B12-nutrients-13-03627
The contents are a compilation of various studies over public forums that are all available to the public via internet search. The information highlighted is intended for information purposes only. Please direct any health concerns to a qualified practitioner of your choice.