The bacteria in the microbiome help digest our food, regulate our immune system, protect against other bacteria that cause disease, and produce vitamins including B vitamins B12, thiamine and riboflavin, and Vitamin K, which is needed for blood coagulation.
Additionally, our microbiome is essential in the innate and adaptive immunity and in turn, the immune system shapes the microbiome.
We are synergy in motion, each system interdependent, mind, body and spirit creating the whole of our entity.
In as much as our microbiome influences our digestion, assimilation and elimination, it is also bidirectionally influenced.
Our body is in a constant state of flux, with each system maintaining its own biochemical feedback to and from organs, cells & tissues to maintain equilibrium.
When the microbiome is our of balance it has a detrimental effect on all our systems. It is a keystone marker for health and well-being and must be kept in pristine condition.
There are so many detrimental influences to upset this delicate balance, from dietary indiscretions, genetic polymorphisms, to bacterial infections and environmental assaults, the list is endless.
We can maintain a healthy microbiome, especially when we educate ourselves in its function.
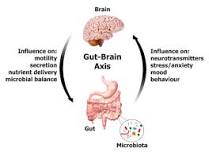
There is an undeniable connection between the gut and the brain via the vagus nerve.
The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis?
“The vagus nerve connects the gut and brain, through the gut-brain axis. It communicates information from the gut to the brain using neurotransmitters (such as serotonin and glutamate) and gut hormones, all of which play a vital role in sleep, mood, pain, stress and hunger.
Preclinical and clinical studies have shown bidirectional interactions within the brain-gut-microbiome axis. Gut microbes communicate to the central nervous system through at least 3 parallel and interacting channels involving nervous, endocrine, and immune signaling mechanisms.
Here we are presented with a review of the role of butyrate, microbiota, and neurological function.
“The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis?
Highlights
*Butyrate is produced by specific bacteria, mainly in the colon, and is taken up by the host.
*Butyrate affects multiple host physiological processes via specific transporters/receptors and as an HDAC inhibitor.
*Supraphysiological doses of butyrate exert potent neuropharmacological effects, facilitating synaptic tagging and capturing.
*Physiological levels of butyrate may influence the brain indirectly via regulating immune system and vagus nerve activity. Microbiota-derived volatile butyrate may be involved in host behaviour including social communication.”
https://doi.org/10.1016/j.neuint.2016.06.011
My obsession with the microbiome and its importance in our health continues.
The role of butyrate and short chain fatty acids in a healthy biome is beyond dispute and there is, a plethora of studies attesting to this.
To recap
 The microbiome is thought to be affected by medications and diet. Studies have also found that people who don’t process insulin properly have lower levels of a certain type of bacteria that produce a type of fatty acid called butyrate.
The microbiome is thought to be affected by medications and diet. Studies have also found that people who don’t process insulin properly have lower levels of a certain type of bacteria that produce a type of fatty acid called butyrate.
-
Butyrate is a short-chain fatty acid produced by your gut microbes when they break down dietary fibre. This metabolite has many important functions within the human body, particularly for digestive health, as well as supporting brain health and protecting against disease.
-
Butyrate is a major short-chain fatty acid produced during gut flora-mediated fermentation of dietary fibers. Legumes (beans, peas, and soybeans), fruits, nuts, cereals, and whole grains are good sources of dietary fibers. Butyrate is also found in butter and cheese.
-
Butyrate is, also termed a prebiotic.
-
Prebiotic foods are rich in dietary fibres. So what you’re looking for are fruit, vegetables, wholegrains, and pulses. Your body can’t digest these fibres, so they travel to your gut where they feed the good bacteria that make butyrate.
-
A specific classification of bacteria, called Firmicutes, are especially renowned for being butyrate producing bacteria.
Foods to increase butyrate:
Almond apple barley Chickpea (bengal gram) garlic kiwifruit maize oat and wheat bran
Here, once again we see the benefit of butyrate
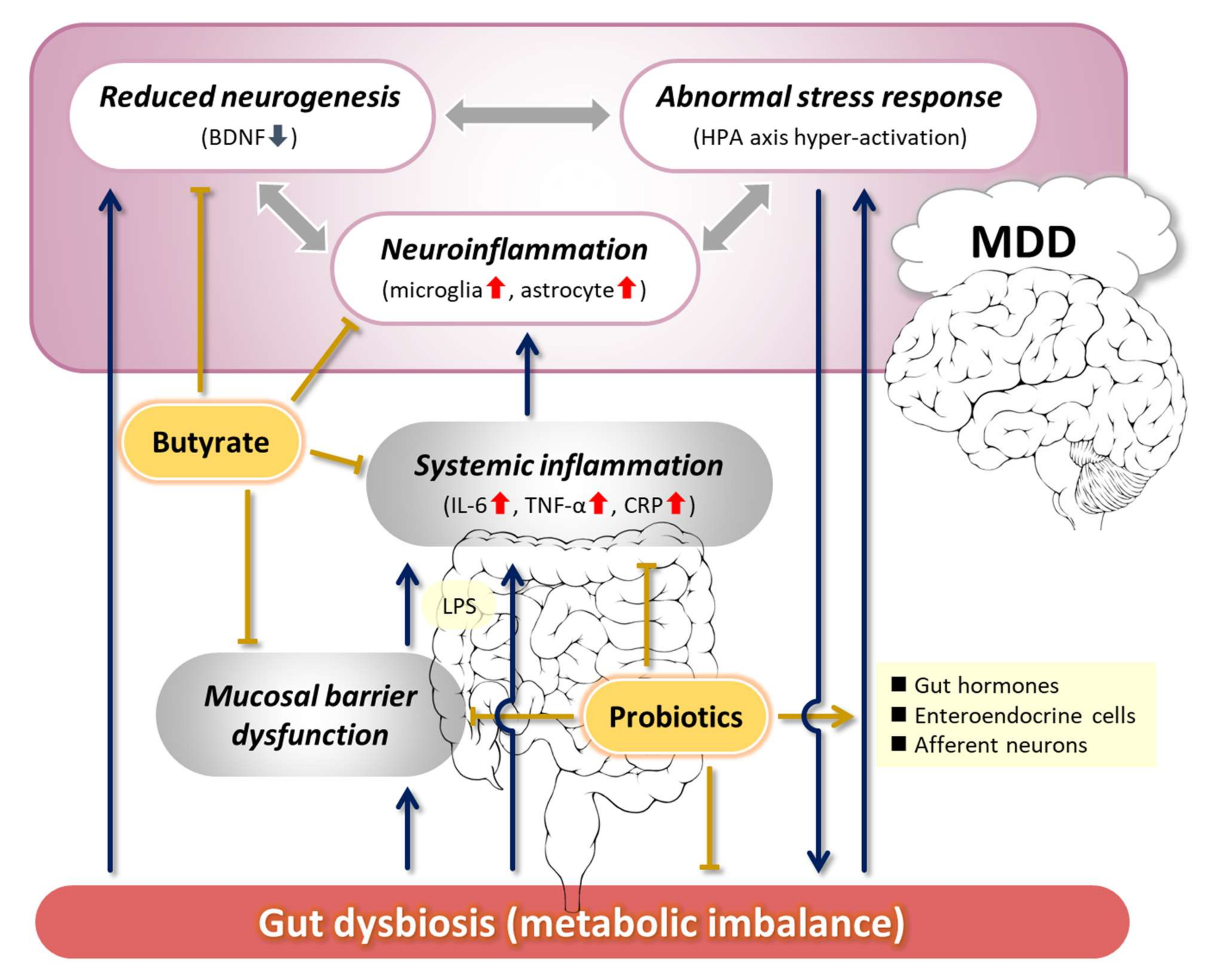
“A delicate and ingenious balance between host and gut microbiota contributes to intestinal homeostasis. Accumulating lines of evidence have proven that gut microbiota-derived antigen-induced aberrant immune responses are initial events of chronic relapsing intestinal inflammation.
Multitudes of studies have elucidated that gut microbiota-derived metabolites participate in the maintenance of intestinal homeostasis as well as in the pathogenesis of IBD
Consistently, oral administration of butyrate markedly ameliorated mucosal inflammation in DSS-induced murine colitis through inhibition of neutrophil-associated immune responses such as pro-inflammatory mediators and NET formation. Our data thus reveal that butyrate constrains neutrophil functions and may serve as a novel therapeutic potential in the treatment of IBD.
We found that butyrate significantly inhibited IBD neutrophils to produce pro-inflammatory cytokines, chemokines, and calprotectins”
https://www.tandfonline.com/doi/full/10.1080/19490976.2021.1968257
 The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems.
The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems.
Studies continue to provide strong evidence of the necessity of a balanced microbiome for optimal health.
People often “feel it in my gut” an inner knowing that can certainly be attributed to the gut/brain connection.
“Strong evidence suggests that gut microbiota has an important role in bidirectional interactions between the gut and the nervous system. It interacts with CNS by regulating brain chemistry and influencing neuro-endocrine systems associated with stress response, anxiety and memory function.
Evidence indicates that microbiota communication with the brain involves the vagus nerve, which transmits information from the luminal environment to CNS.
“The enteric microbiota is distributed in the human gastrointestinal tract and, although each person’s microbiota profile is distinct, relative abundance and distribution along the intestine of these bacterial phylotypes is similar among healthy individuals. The two more prominent phyla are Firmicutes and Bacteroides accounting for at least ¾ of the microbiome [4]. This microbial community has important metabolic and physiological functions for the host and contributes to its homeostasis during life.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4367209/
 Various bacteria identified showed a potential involvement in the way people produce neurotransmitters, particularly those linked to depression such as glutamate.
Various bacteria identified showed a potential involvement in the way people produce neurotransmitters, particularly those linked to depression such as glutamate.
Do gut bacteria play a role in depression?
Could the gut microbiota influence depression symptoms?
Depression is a common mental health disorder and a leading cause of disability around the world, according to the World Health Organisation. Trusted Source (WHO).
Research Trusted Source shows gut microbiota may play a role in depressive disorders and that depressive symptom levels vary across ethnic groups.
Now, researchers from the United Kingdom and the Netherlands have demonstrated that 13 types of bacteria found in the gut are associated with depression symptoms, namely “Eggerthella, Hungatella, Sellimonas, and Lachnoclostridium are more abundantly found, while Coprococcus, Lachnospiraceae UCG001, Ruminococcusgauvreauii group, Eubacterium ventriosum, Subdoligranulum, Ruminococcaceae (UCG002, UCG003, UCG005), and [the] family Ruminococcaceae were less abundant in individuals with higher symptoms of depression,” according to Dr. Najaf Amin.
“Namely, these gut species, Eggerthella and Eubacterium ventriosum, have been shown to produce butyrate — an important precursor molecule to GABA, a brain chemical that functions in regulatory control of glutamate,” he explained.
(credit: Paul Ian Cross, PhD on December 12, 2022 — Fact checked by Anna Guildford, Ph.D.)
https://www.medicalnewstoday.com/articles/do-gut-bacteria-play-a-role-in-depression
Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve
“Data with L. rhamnosus (JB-1) suggest that nonpathogenic bacteria can modulate the GABAergic system in healthy mice and therefore may have beneficial effects in the treatment of depression and anxiety.” suggesting that there is therapeutic potential of bacteria in modulating brain and behavior.
Stress-induced changes to the microbiome may in turn affect the brain and behavior. A few studies suggest that defensive molecules the gut produced during infection, called inflammatory cytokines, disrupt brain neurochemistry and make people more vulnerable to anxiety and depression.
Short Chain Fatty Acids (SCFAs) communicate with cells which produce serotonin, a neurotransmitter (and a hormone) that regulates your mood, as well as levels of anxiety and happiness. Basically, your gut microbes can help your body produce more serotonin.
‘Microbes, Oxytocin, and Healthful longevity’
Susan E Erdman, 2014
“Together this prevents over-reactivity to self or environmental factors that otherwise lead to premature death.”
Landmark 2011 citation:
Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve
‘Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve’. There is increasing, but largely indirect, evidence pointing to an effect of commensal gut microbiota on the central nervous system (CNS).
However, it is unknown whether lactic acid bacteria such as Lactobacillus rhamnosus could have a direct effect on neurotransmitter receptors in the CNS in normal, healthy animals.
GABA is the main Central Nervous System inhibitory neurotransmitter and is significantly involved in regulating many physiological and psychological processes.
Alterations in central GABA receptor expression are implicated in the pathogenesis of anxiety and depression, which are highly comorbid with functional bowel disorders. In this work, we show that chronic treatment with L. rhamnosus (JB-1) induced region-dependent alterations in GABAB1b mRNA in the brain with increases in cortical regions (cingulate and prelimbic) and concomitant reductions in expression in the hippocampus, amygdala, and locus coeruleus”
https://www.jstor.org/stable/41352392
Interesting research, albeit in mice of the ability of lactobacillus to activate the vagus nerve and positively improve brain function.
“We have begun to decipher the mechanism by which a gut microbe modulates brain function and behaviors. This could be key in the development of new more effective therapies,” Costa-Mattioli said. “Indeed, we think that our findings have strengthened the rather unconventional idea that it might be possible to modulate specific behavior through the gut microbiome using select bacterial strains.”
It is known that when the vagus nerve is active, it releases oxytocin, a hormone that promotes social interaction. Oxytocin is released into the reward areas of the brain where it binds to molecules called oxytocin receptors, triggering ‘social reward.'”
https://medicalxpress.com/news/2018-12-microbial-based-treatment-reverses-autism-spectrum.html
Palmitoylethanolamide: A Natural Compound for Health Management
“PEA’s ability to modulate gut health may have an impact on the CNS indirectly [84]. The gut–brain axis (GBA) describes the bidirectional communication between the central and enteric nervous system, linking cognitive and emotional centers of the brain with intestinal function [116]. Maintaining microbial symbiosis and gut barrier integrity is considered crucial for adequate brain development and neurological functioning [117]. “Leaky gut” caused by chronic intestinal inflammation [118], is likely associated with neuropsychiatric and neurodegenerative disorders via intestinal cytokine formation “. “PEA may contribute to correcting the effects of dysbiosis. In an induced inflammation state, such as vitamin D deficiency in mice, intraperitoneal administration of PEA increases the level of commensal bacteria such as Akkermansia muciniphila (protective effects against obesity and diabetes), Eubacterium (microbiome regulatory properties) and Enterobacteriaceae [83]. Moreover, exogenous administration of PEA relieves chronic and acute GIT inflammation via its action on PPAR-α in the colon [84]. For these reasons, PEA is becoming an increasingly popular topic in microbiome research.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8157570/
“Akkermansia muciniphila is a mucin-degrading bacterium commonly found in human gut. A. muciniphila has been inversely associated with obesity, diabetes, inflammation, and metabolic disorders. Due to its highly promising probiotic activities against obesity and diabetes, A. muciniphila has drawn intensive interest for research and development in recent years. A number of human and animal studies have shown that the abundance of A. muciniphila in the gut can be enhanced through dietary interventions.
Available evidence from animal studies showed that viable A. muciniphila or prebiotics (FOS) was able to consistently promote A. muciniphila abundance in the gut, suggesting a great potential for future development of dietary intervention approaches using viable bacterium or FOS for increasing gut A. muciniphila. Supplementation of B. animalis could also increase A. muciniphila by producing SCFA and facilitating mucin growth to feed the bacterium.
Dietary polyphenols are inconsistent, cranberry extract and Concord grape polyphenols are active but green tea and whole grape showed no effect. The inconsistency may be related to their difference in polyphenol profile but to identify the active polyphenols is challenging due to their abundance and diversity in the extract. It should also be noted that to maintain A. muciniphila abundance in the gut one may want to avoid high-fat diet and heavy alcohol consumption, though the results were based on the measurement of relative abundance of gut microbials.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6223323/
 A new look at MS and our microbiome
A new look at MS and our microbiome
“Blocking the activity of the reactor called the aryl hydrocarbon receptor in T cells resulted in both a decrease in inflammation and recovery in mouse models of multiple sclerosis.””Ultimately, fine-tuning the immune response using the microbiome could save patients from dealing with the harsh side effects of immunosuppressant drugs.”
Gaultier and his collaborators blocked the activity of the regulator, called “aryl hydrocarbon receptor” in immune cells called T cells, which led to a dramatic effect on the production of bile acids and other metabolites in the microbiomes of lab mice. With this receptor out of commission, inflammation decreased and the mice recovered.”