 Our research team continually strives to bring you the most current research to enable you, the reader to understand the importance of a healthy immune system and the role that our gut function has to play in it. Here we present our latest findings on innate immunity, cells involved in our innate protection and how you are able to improve your resilience and resistance.
Our research team continually strives to bring you the most current research to enable you, the reader to understand the importance of a healthy immune system and the role that our gut function has to play in it. Here we present our latest findings on innate immunity, cells involved in our innate protection and how you are able to improve your resilience and resistance.
The kinetics of immune responses in relation to clinical and virological features of a patient with mild-to-moderate coronavirus disease 2019 (COVID-19) that required hospitalisation has been published by Melbourne researchers at the University of Melbourne.
This research is instrumental in our ability to understand the important role that innate immunity plays in protecting us from invasion by viruses, bacteria and other foreign assailants that cause debilitating symptoms disrupt our health and as seen in the latest Covid-19 Pandemic, ultimately, our lives.
The current consensus is to find a vaccine to combat this newly acquired virus infection.
Results from various studies prove conclusively that by strengthening our innate immunity and building a robust resilience is paramount to a reduction in symptoms and duration of illness.
Researchers have mapped immune responses from one of Australia’s first novel coronavirus (COVID-19) patients, showing the body’s ability to fight the virus and recover from the infection.
Professor Katherine Kedzierska leads research at the Peter Doherty Institute for Infection and Immunity that discovers how the human body overcomes coronavirus.
Researchers at the Peter Doherty Institute for Infection and Immunity (Doherty Institute) – a joint venture between the University of Melbourne and the Royal Melbourne hospital –
were able to test blood samples at four different time points in an otherwise healthy woman in her 40s, who presented with COVID-19 and had mild-to-moderate symptoms requiring hospital admission.
A 47-year-old woman from Wuhan, Hubei province, China, presented to an emergency department.
(https://biomedicalsciences.unimelb.edu.au/news-and-events/covid-19-the-immune-system-can-fight-back in Melbourne, Australia.).
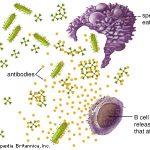
It was reported that increased antibody-secreting cells (ASCs), follicular helper T cells (TFH cells), activated CD4+ T cells and CD8+ T cells and immunoglobulin M (IgM) and IgG antibodies that bound the COVID-19- causing coronavirus SARS-CoV-2 were detected in blood before symptomatic recovery.
These immunological changes persisted for at least 7 d following full resolution of symptoms.
WHAT ARE CD cells?
CD8+ (cytotoxic) T cells, like CD4+ Helper T cells, are generated in the thymus and express the T-cell receptor. CD8+ T cells (often called cytotoxic T lymphocytes or CTLs) are very important for immune defence against intracellular pathogens, including viruses and bacteria, and for tumour surveillance.
In humans, the CD4 protein is encoded by the CD4 gene.
CD4+ T helper cells are white blood cells that are an essential part of the human immune system.
They are often referred to as CD4 cells, T-helper cells or T4 cells. They are called helper cells because one of their main roles is to send signals to other types of immune cells, including CD8 killer cells, which then destroy the infectious particle. If CD4 cells become depleted, for example in untreated HIV infection, or following immune suppression prior to a transplant, the body is left vulnerable to a wide range of infections that it would otherwise have been able to fight.
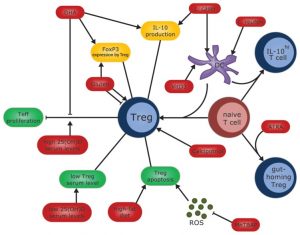
PNAS Proceedings of the National Academy of Sciences of the United States of America report CD4+CD25+ regulatory T cells (Treg) play a crucial role in the regulation of immune responses.
CD4+CD25+Foxp3+ regulatory T cells (Treg) maintain immune homeostasis by limiting T-cell responses to self-, environmental-, and pathogen-associated antigens and by modulating innate immune responsiveness.
Alice McNally et.al reported on regulatory T cells being important controllers of adaptive and innate immune responses.
Influence of Dietary Components on Regulatory T Cells.
Common dietary components including vitamins A and D, omega-3 and probiotics are now
widely accepted to be essential to protect against many diseases with an inflammatory nature.
Shohreh Issazadeh-Navikas, et.al note that CD4+CD25+FoxP3+ Tregs play a central role in maintaining peripheral immune tolerance. As such, it is not surprising that proper functioning of Tregs is important in gut-associated immunity. Even more understandable, she notes is why the critical gut-associated immune cell population, Tregs, adapts itself to the influence of recurrent ingested dietary components such as Vitamin A and Vitamin D, gluten and fatty acids.
Additionally, as functional food containing probiotics become more widely available and popular, understanding the mode of action of these dietary factors on regulation of Treg homeostasis could prove valuable to control many chronic inflammatory conditions directly affecting the gut regulated by Tregs.
(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3276397/)
Animal studies have demonstrated that the gut microbiota can regulate the population of CD4+CD8-CD25+ and CD4+CD8+CD25+ T cells.
(https://www.researchgate.net/publication/325577516_Regulation_of_CD4CD8-CD25_and_CD4CD8CD25_T_cells_by_gut_microbiota_in_chicken)
Simone A. Joosten et.al demonstrated that Regulatory T cells (Treg) comprise multiple subsets and are important in controlling immunity and inflammation. Studies found that CD8+ Tregs were only found in primed but not in naive donors, suggesting that they are induced in vivo. The results of this study concluded that CD+, LAG-3, CD25+, FoxP3 +CCL4, Treg subset thus may play a role in immunoregulation in humans, including infectious diseases.